Product Description
| Download Documents | |
| Product Sheet | |
| SDS | COA_Lot#NB0350631F14 |
CellMosaic’s Phenyldiazirine oxLink™ (T2A10) is a proprietary photo-crosslinking reagent developed at CellMosaic® for studying biomolecular interactions.
This reagent combines the highly efficient carbene-generating phenyldiazirine group with convenient oxime reversible chemical crosslinking and the super hydrophilic AqueaTether™ (AqT™) linker. These structural features make it highly advantageous to use this reagent over traditional photo-crosslinking reagents.
- Trifluoromethyl phenyldiazirine (TFMP) group: TFMP photolyzes around 360 nm, at which photodamage to biomolecules is minimized. The generated carbene inserts C–H bonds into the neighboring biomolecular partner within picoseconds. Because the electron-withdrawing trifluoromethyl group confers stability on the intermediate diazo-isomer, no side products are detected under normal labeling conditions.
- Aminooxy group for chemical crosslinking of any biomolecule containing a ketone or aldehyde group. Aminooxy-ketone ligation is orthogonal to peptide chemistry and will allow highly selective solid phase-based separation of the aminooxy-labeled crosslinked products. In particular, the selective purification method will permit the characterization of protein complexes in complex matrices, such as plasma, cellular membranes, and cell lysates, where these samples may contain free thiols that interfere with the labeling and purification processes. Figure 1 shows a workflow of how a reversible oxLink™ can be used to crosslink the interacting biomolecule partner and identify the crosslinking site or detect the interaction partner.
- Hydrophilic AqT™ linkers: TFMP group is highly hydrophobic, biomolecules labeled with a TFMP using a traditional ethylene and ethylene glycol-type linker tend to aggregate and destabilize the labeled protein. T2A10 AqT™ linker is made by linking two threitols together, greatly increasing the hydrophilicity of oxLink™ (Figure 2) and its water solubility (>27 mg/mL). The molecule is also more biocompatible with decreased non-specific hydrophobic interactions with other biomolecules, allowing high loading of phenyldiazirine groups.
The product is sold as a lyophilized powder in 1 vial of 250 µg (Cat# CM81402-250UG) or 4 vials of 250 µg (Cat# CM81402-4x250UG).
Chemical Information
- Chemical name: Phenyldiazirine sxLink™ (T2A14)
- Chemical formula: N/A
- Molecular weight: 633.66 Da CAS number: N/A
Specification
- Physical appearance: white lyophilized powder in a 1.5 mL centrifuge tube
- Storage temp.: -20 °C
- Purity: ≥ 95 % by HPLC
Application of the Product
- Labeling biomolecules containing aldehyde or ketone groups
- Studying dynamic biomolecule interactions via photo-crosslinking (e.g., studying biological complexes and networks, protein-protein interactions, protein-DNA interactions, small ligand-protein interactions)
Key Features of the Product
- Highly water soluble (>27 mg/mL) and biocompatible
- High loading with minimized aggregation
- Contains orthogonal peptide chemical crosslinking group capable of forming a reversible linkage
- Contains an efficient carbene-generating photo-crosslinking group
Figure 1. oxLink™ workflow using water-soluble Phenyldiazirine oxLink™ (T2A10)
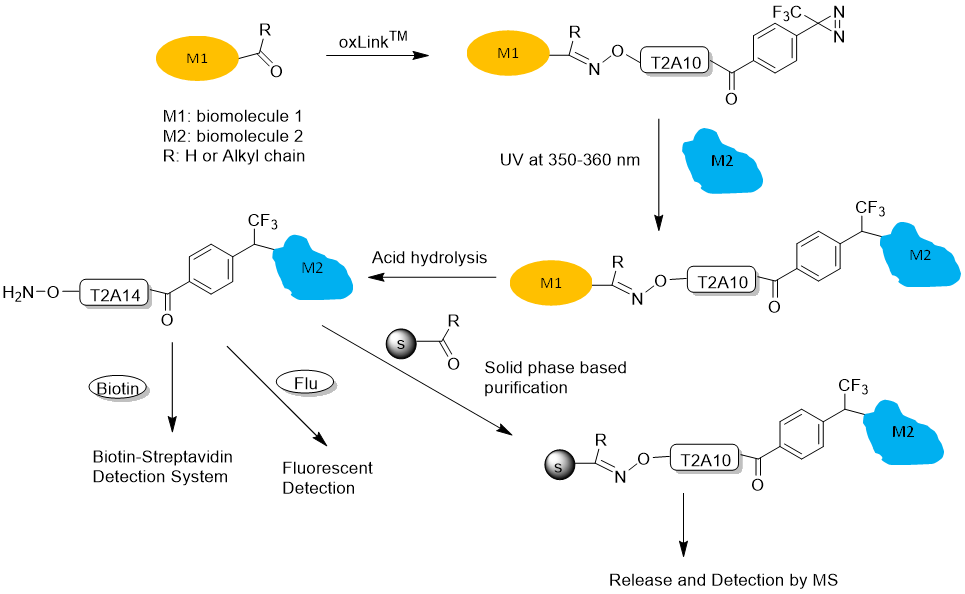
Figure 2. C18 HPLC analysis of 4-[3-(trifluoromethyl)-3H-diazirin-3-yl]benzoic acid (TFDB, red, in DMSO) and oxLink™ (blue, in water).
![oxlink-hplc-data.png C18 HPLC analysis of 4-[3-(trifluoromethyl)-3H-diazirin-3-yl]benzoic acid (TFDB, red, in DMSO) and oxLink™ (blue, in water).](https://cdn2.bigcommerce.com/server1500/02cc5/product_images/uploaded_images/oxlink-hplc-data.png)
References
Protein crosslinking reviews: a) Brunner, J. (1993) Annu. Rev. Biochem. 62, 485−514. b) Freedman, R. B. (1979) Trends Biochem. Sci. 193–197. c) Herrmann, J. M., Westermann, B., Neupert, W. (2001) Methods Cell Biol. 65, 217–230. d) Fancy, D. A. (2000) Curr. Opin. Chem. Biol. 4, 28–32. e) Fasold, H., Klappenberger, J., Meyer, C., Remold, H. (1971) Angew. Chem. Internat. Edit. 10, 795–801. f) Bayley, H. Photogenerated Reagents in Biochemistry and Molecular Biology, Vol. 12. Elsevier, Amsterdam, Neth, 1983.
3-Trifluoromethyl-3-phenyldiazirine reference: Brunner, J., Senn, H. & Richards, F. M. (1980) J. Bio. Chem. 255, 3313−3318.