Product Description
This routine synthesis service is designed for the small-scale preparation of purified and characterized antibody–oligo conjugates (AOCs), with an average of 1–3 oligos per antibody. These multiple-oligo–loaded AOCs are especially suitable for targeted delivery of large amounts of oligos while retaining antibody binding activity. They can also be used for diagnostic applications that require enhanced sensitivity. For routine synthesis of single-labeled AOCs, please see RS0004.
The customer provides the antibody to CellMosaic. An amine-modified oligo can be supplied by the customer or purchased by CellMosaic if the sequence is provided. CellMosaic delivers the final conjugate along with SEC-HPLC analysis data.
Customers can order online by providing oligo information and selecting from various reaction scales, linker types (stable or releasable), and additional optional services.
What's included in the Base Price:
- Labor and materials (excluding antibody and oligo).
- Initial QC and quantitation of the starting antibody by SEC HPLC, and of the oligo by C18 HPLC to check the purity.
- In-process monitoring of the oligo modification and antibody-oligo conjugation.
- Default filtration and cartridge type purification.
- Final AOC analysis by SEC HPLC to determine purity, aggregation profile, concentration, percentage of unreacted antibody, residual oligo, and multiple AOC species (if separation is sufficient).
- Use of sterilized buffers and plasticware throughout preparation.
Delivery: Final product is supplied in PBS buffer in a single tube along with a COA that includes SEC-HPLC data, concentration, and oligo loading.
Timeline: Approximately 2–3 weeks after receiving the starting antibody and oligo.
Selections and Add-Ons:
1. Reaction Scale: Choose between 1 mg, 3 mg, and 5 mg antibody labeling scale. Typical recovery is 30-60%, depending on antibody and oligo properties (note: HPLC purification may reduce yield to 20-50%). If you do not see the desired scale, please contact us for a custom quote.
2. Oligo Length: Please indicate the number of nucleotides in your oligo so that appropriate handling and conditions can be applied.
3. Linker: Choose from stable maleimide-thiol or releasable VC-PAB linker. if you require a different linker, please contact us for a quote.
4. Antibody Labeling Sites: Choose from surface amines or reduced thiols at hinge region. Note: hinge region thiol labeling may results in a higher percentage of unlabeled antibody compared to surface amine chemistry. AEX HPLC purification is recommended for this option.
5. Optional AEX HPLC analysis: AEX-HPLC provides charge-based analysis of AOCs and often offers better resolution. If conjugate peaks are separable, AEX can be used to calculate overall oligo loading. Three samples are generally required for this analysis (oligo, antibody, and AOC). [Click here for more information].
6. Optional AEX HPLC purification: For project requires complete removal of unreacted antibody and oligo, AEX HPLC is highly effective. After purification, AOCs will be buffer-exchanged into 1xPBS buffer and reanalyzed by SEC HPLC to determine the concentration.
7. Optional 0.2 µm Sterile Filtration: Routine AOC preparation at CellMosaic uses sterilized buffers and pyrogen-free consumables. The final purified product is suitable for in vitro and in vivo studies. However, customers may request additional 0.2 µm sterile filtration after purification (note: this step may slightly reduce the final yield).
Requirements for Antibody:
- IgG Type: Full length IgG (MW of 150KDa), IgM, or F(ab')2 (110KDa). For other antibody fragments or protein oligo conjugation, please contact us for a quote.
- Purity: preferably >90% pure by gel electrophoresis, with minimal aggregation (Lower purity will not prevent AOC preparation but may affect final product quality).
- Amount: The supplied antibody should exceed the selected reaction scale by several hundred micrograms (based on UV protein quantitation). Approximately 20–50 µg of antibody is required for initial QC and optional analyses. If a repeat reaction is necessary, additional antibody must be provided by the customer. Any unused material can be returned upon request.
- Information: Please provide as much antibody information as possible — amount supplied, molecular weight, IgG type, etc. Attach any available QC documentation.
Requirements for Amine Oligo:
- Type: Standard amine DNA oligo with deoxyribose–phosphate backbone. For modified backbones such as phosphorothioate (PS), locked nucleic acids (LNA), RNA, or morpholino oligos, please contact us for a quote.
- Purity: HPLC-purified and lyophilized amine oligo with ≥90% amino-oligo content by C18-HPLC. No additional components containing primary/secondary amines or thiol groups should be present.
- Amount:
-
1 mg antibody labeling → >70 nmol oligo
-
3 mg antibody labeling → >200 nmol oligo
-
5 mg antibody labeling → >350 nmol oligo
(Measured by UV.) Unused materials can be returned upon request.
-
- Information: Provide as much detail as possible — amount, molecular weight, sequence, extinction coefficient — and attach QC data from your oligo vendor. Sequence information is required if CellMosaic is to order the oligo.
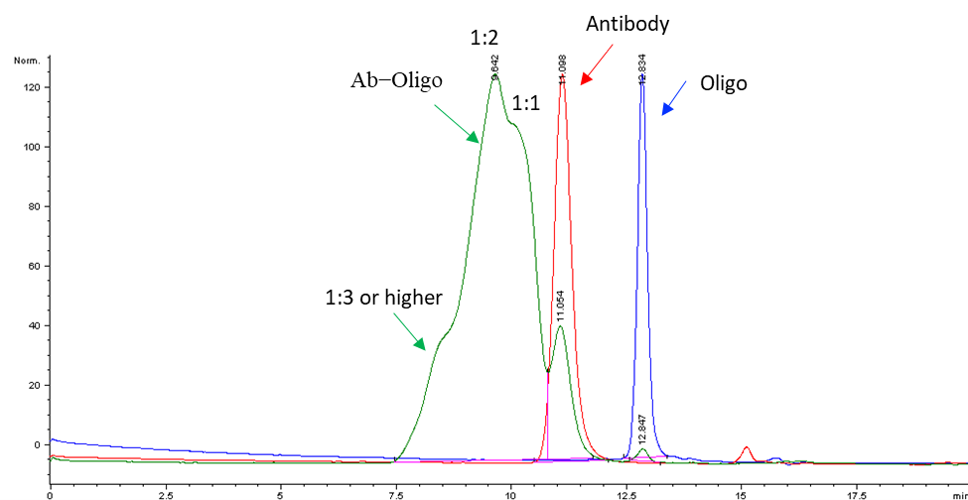
An Example of AOCs Prepared at CellMosaic
Antibody: Trastuzumab biosimilar Human anti-Her2 mAb (CM51000) Oligo Length: 18mer oligo with 5’ NH2-C6 modification
Reaction Scale: 1 mg of antibody Linker: stable maleimide-thiol Antibody Labeling Sites: Surface amines
Purification Method: Default filtration and cartridge type (no AEX HPLC purification)
Specification of the final AOCs:
Calculated average degree of labeling (DOL): 1.9 Unreacted antibodies: 9.3%
Unreacted oligo: ~0.5% Conjugate Purity: >90% (85% recovery)
Figure 1: Size-exclusion chromatography (SEC) HPLC analysis of the unlabeled antibody (Ab) (red trace), oligo (blue trace), and purified AOCs (green trace).