There are no products listed under this category.
Welcome to CM Online Store!
Antibody Drug Conjugate Kits!
AqT bioconjugates coming soon!
The modification and conjugation of nucleic acids and oligonucleotides is quite different from other biopolymers, as they are rather inert to common bioconjugation reagents and conditions. Although there are reagents that can modify the base units, base modification may affect the binding capacity or propensity of nucleic acids and oligonucleotides. Many biopolymers cannot withstand the final basic cleavage conditions of solid phase oligo synthesis, so oligo bioconjugation usually is done in solution after oligo synthesis. On the other hand, small molecules may be incorporated into the oligo during oligo synthesis. Oligos are generally modified first with extra functional groups suitable for bioconjugation at the 5’ or 3’ end during oligo synthesis.
CellMosaic offers versatile oligonucleotide labeling and conjugation strategies, depending on the application of the product. Usually a single pure labeled oligo can be obtained. The advantages of our oligo labeling strategy are:
Oligo modification through an amino functional group is the most straightforward labeling method that often used at CellMosaic. Aliphatic primary amine can be introduced to 5' or 3' end of the oligo during synthesis. Exo-cyclic amino groups in Cytosine and Guanine are usually not modified under the reaction conditions. An amino group can also be introduced to the 5'-phosphate via carbodiimide coupling. Oligo modification through a thiol functional group is another straightforward labeling method that used at CellMosaic®.
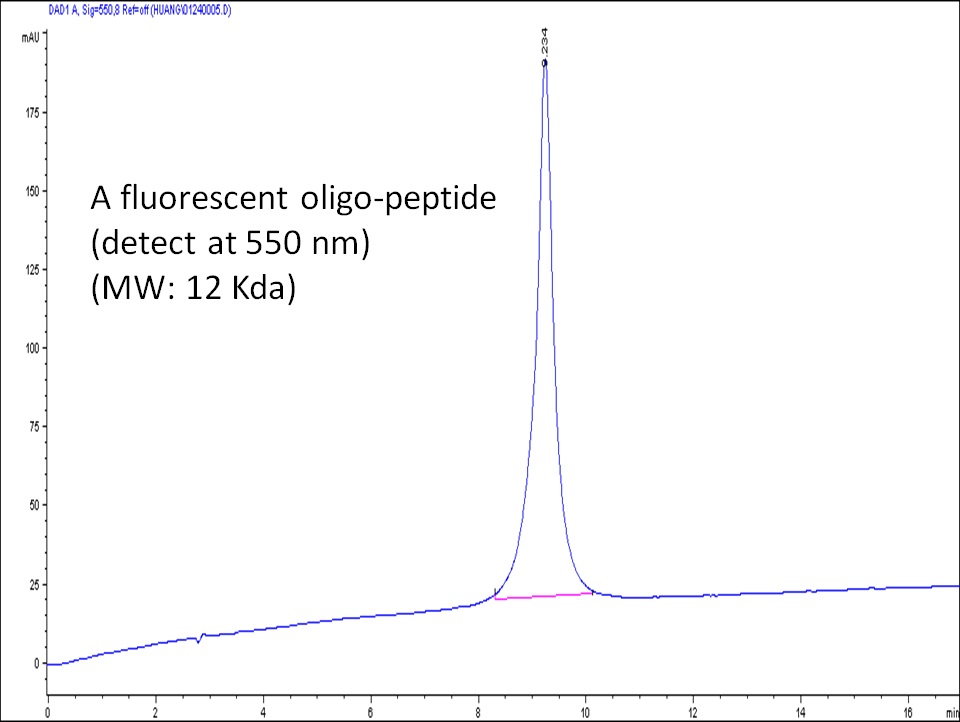
Example 1. HPLC analysis of an oligo-peptide conjugate synthesized at CellMosaic through a thioether bond.
Selected Examples:
There are no products listed under this category.