AqueaTether™ (AqT™) Technologies
| AqT™ Reagents and Kits |
CellMosaic is developing next-generation bioconjugation kits and products based on its proprietary super-hydrophilic and high-loading AqT® linkers. AqT® linkers are novel proprietary biomaterials with innovative chemical architectures invented at CellMosaic based on a class of natural and edible sugar alcohol compounds. AqT® molecules are designed for labeling and conjugating biomolecules with very hydrophobic small molecules, such as fluorescent compounds, biotin, and small-molecule drugs. Branched AqT® crosslinking reagents can also be designed to allow high amounts of detecting molecules to be loaded onto a biomolecule for detection purposes.
The AqT® platform can also be applied to create new and better therapeutics across a broad range of drug molecules and treatment indications. The AqT® platform can significantly accelerate the drug discovery process to deliver an optimized entity for development. It provides a broadly applicable approach to developing a new chemical entity (NCE) or new biological entity (NBE) with optimized drug profiles. The AqT® platform offers a simple solution to complicated bioavailability, stability, and/or toxicity issues faced by many small-molecule hydrophobic drugs, as well as with large-molecule biologics. For AqT™ technologies in the application of drug development, please visit our website at www.aqttherapeutics.com
Structure of AqT® Linkers
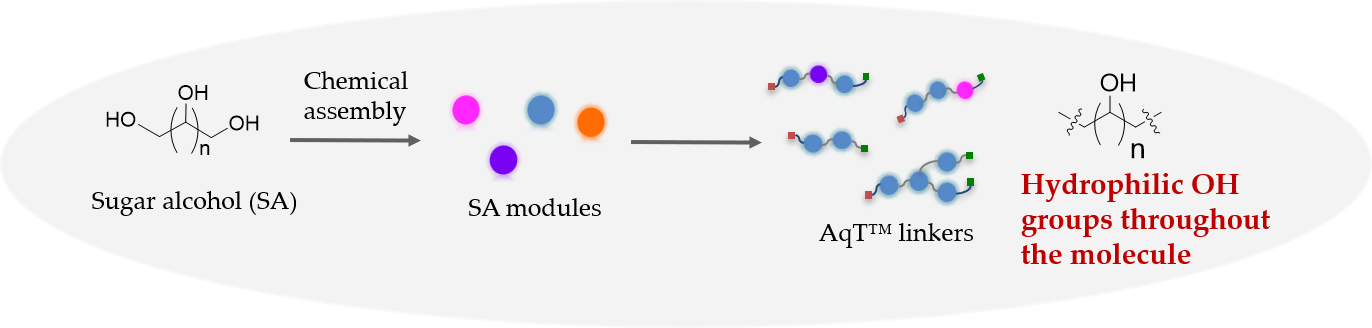
Sugar molecules are probably the most hydrophilic natural compounds to date due to their hydrogen bonding capability (donor and acceptor). Sugar alcohol, also known as polyol, is a hydrogenated form of carbohydrate in which the carbonyl group (aldehyde or ketone) has been reduced to a primary or secondary OH group. Sugar alcohols occur naturally in foods and come from plant products, such as fruits and berries. Sugar alcohols are often used as ingredients in sweeteners and for making polymers such as hydrogel. CellMosaic has established methods to assemble highly sophisticated AqT® architectures from sugar alcohol monomers. Methods have also been developed to convert the OH groups of sugar alcohol to other crosslinking groups, such as aminooxy, maleimide, halide, azide, cyclooctyne, halide, ketone, thiolester, and N-hydroxysuccimide ester (NHS). Table 1 list some popular sugar alcohols that can be used for making AqT™ linkers.
| 3- Carbon | 4- Carbon | 5-Carbon | 6-Carbon |
| Glycerol | Erythritol | Arabitol | Mannitol |
| Threitol | Xylitol | Sorbitol | |
| |
Ribitol | Galactitol | |
| Fucitol | |||
| Iditol | |||
 |
Table 1: Sugar alcohols that can be used for making AqT™ crosslinkers.
Advantages of AqT® Linkers
The advantages of AqT® linkers over traditional linkers and polydisperse carrier polymers are numerous:
1) Super-hydrophilicity can be used to modulate molecular properties after conjugation. AqT® linkers are by far the most hydrophilic molecules in development.
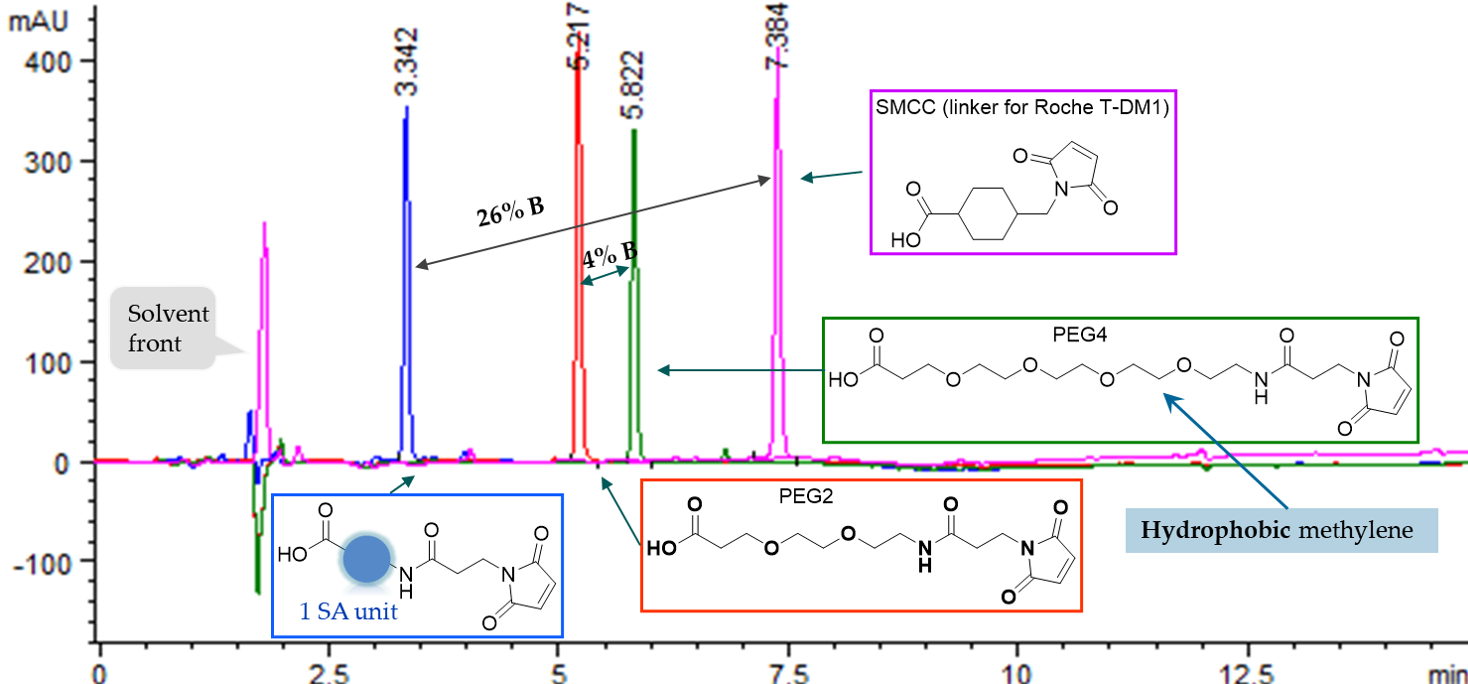
Figure 1. C18 HPLC analysis of AqT™ linker and other commercial linkers.
2) The ability to make crosslinking reagents with versatile functional groups. AqT® linkers are by far the most versatile and tunable molecules in development.
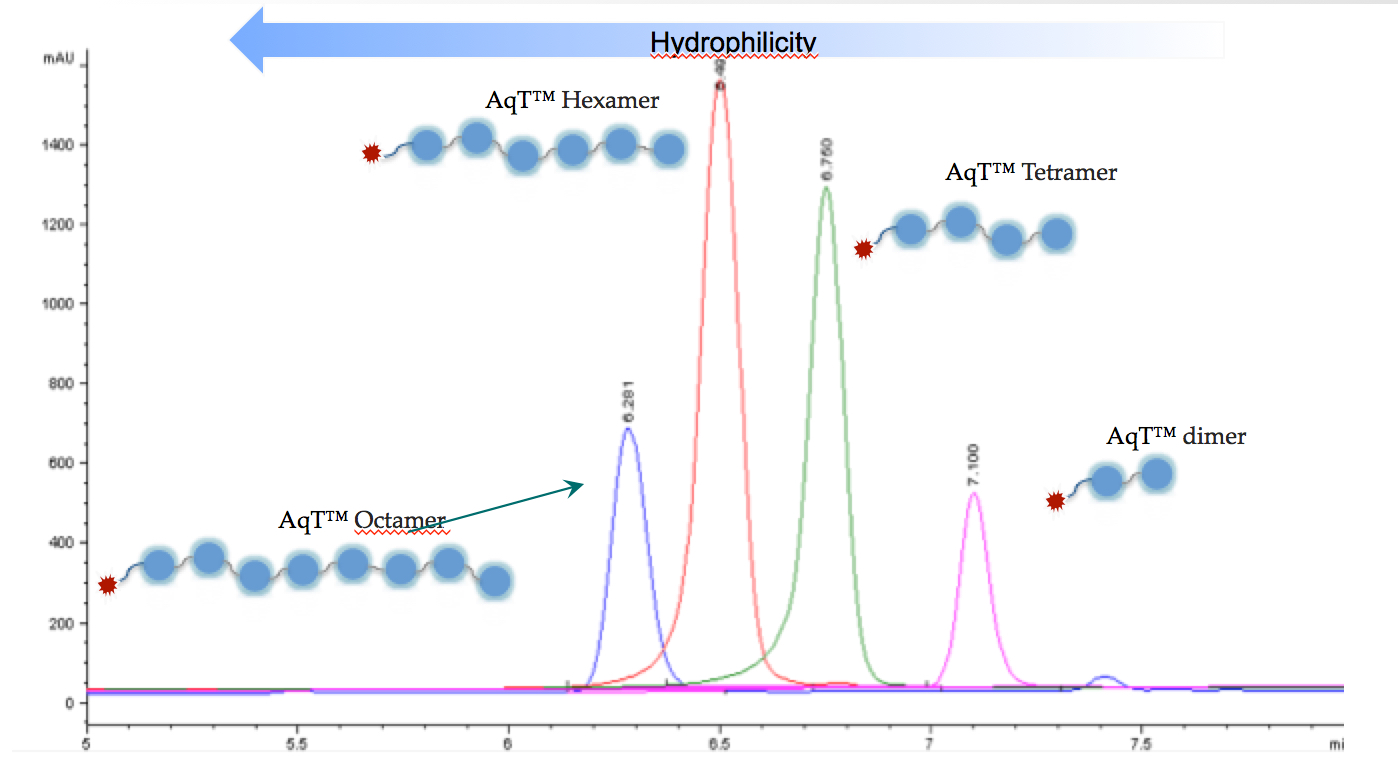
Figure 2. C18 column HPLC analysis of mono-dispersed AqT™ molecules of various SA units.
3) AqT® crosslinker can be used to increase the water solubility of any hydrophobic compound so that conjugation can be done in aqueous buffer
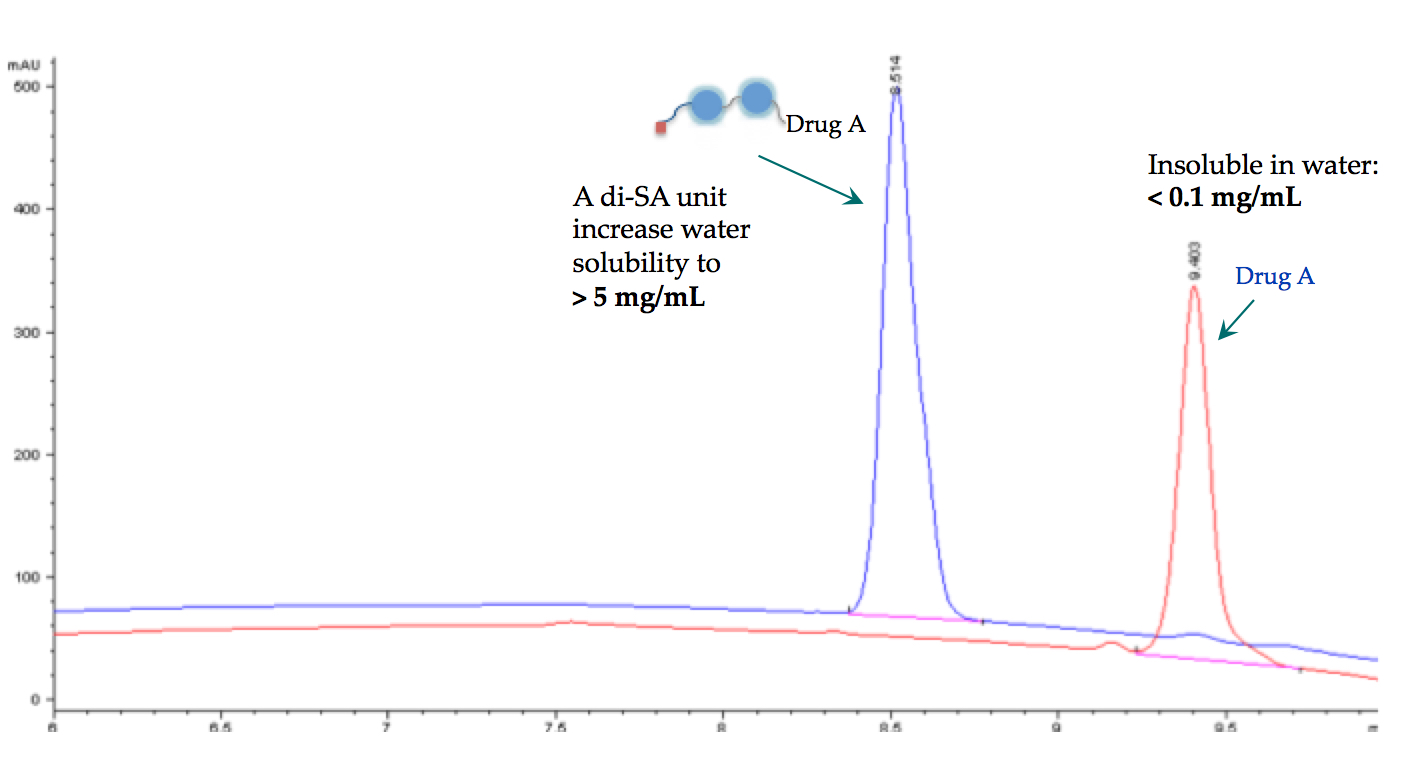
Figure 3. C18 HPLC analysis of a hydrophobic drug before and after di-SA unit AqT™-linker labeling
4) Biocompatible: Biopolymer Properties Unchanged after AqT™ Mediated Labeling and Conjugation with Small Molecules
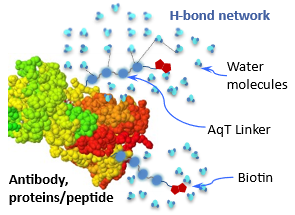 |
Biopolymer such as antibody, protein/peptide labeled with hydrophobic small molecules tend to aggregate and precipitate out from solution over time. AqT™ linker is used to modify these small molecules to increase its hydrophilicity and water solubility. The hydroxy (-OH) groups of AqT™ form a network of hydrogen bonds (H-bond) with neighboring water and create a microenvironment that shields neighboring small molecules or crosslinking moieties from stacking or interacting with one another. A biopolymer can be labeled with a high amount of AqT™-small molecule with no change to its properties. This AqT™ enhanced H-bond network also protects the polymers from enzymatic degradation and retains its activities.
- AqT™ linker retains the properties of biopolymer
- AqT™ technologies are true biocompatible for any biopolymer
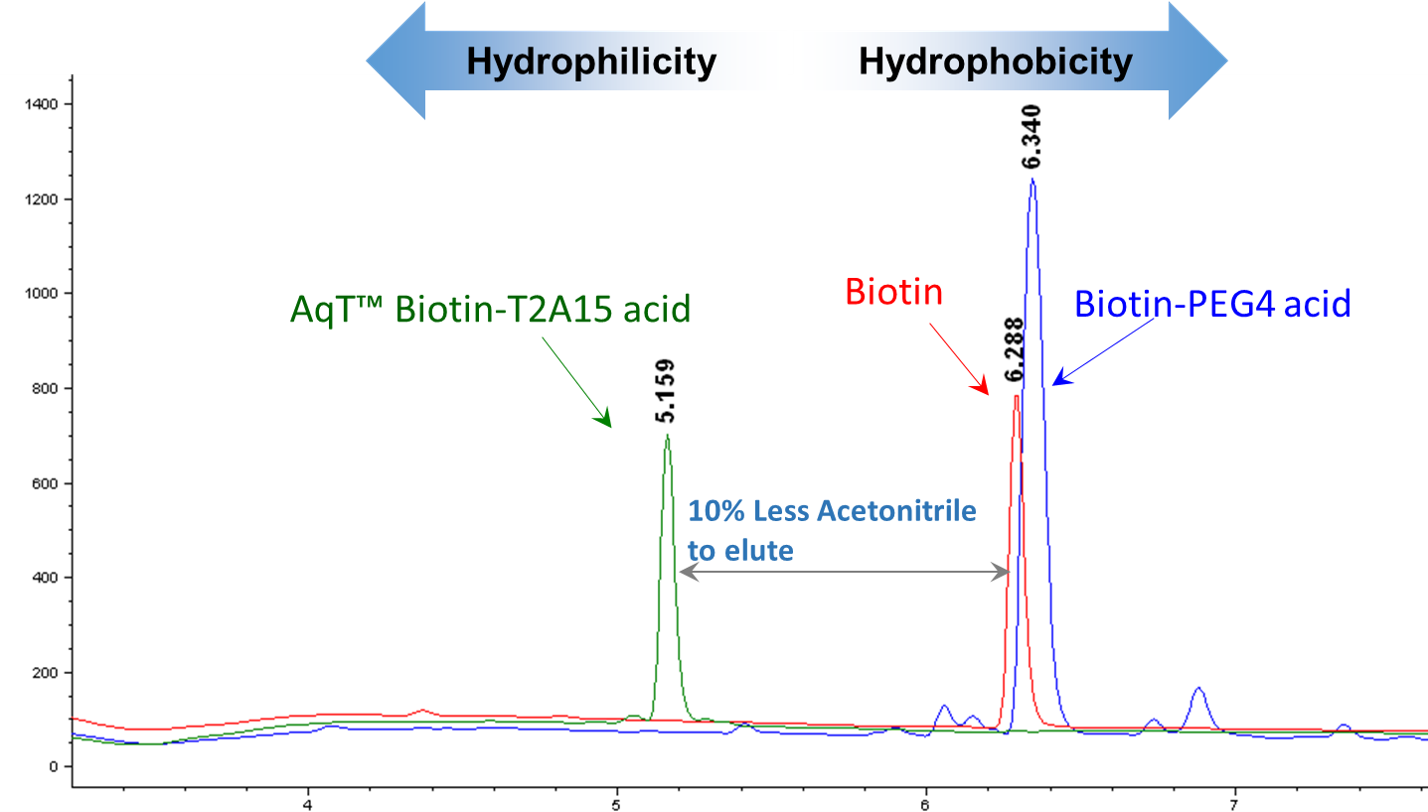
Examples: Antibody biotinylation using AqT™ T2A15 linker (see HPLC data in Figure 4, 5, and 6).
AqT™ T2A15 linker is composed of two threitols and 15 atom lengths. This atom length is equivalent to four polyethylene glycol modified biotin (PEG4) (16 atom lengths) except PEG4 does not increases any hydrophilicity of the biotin.
|
|
Figure 4. C18 HPLC analysis of biotin, AqT™ biotin, and biotin-PEG4. AqT™ Biotin: less hydrophobic
Biotin and PEGylated biotin: very hydrophobic
|
|
|
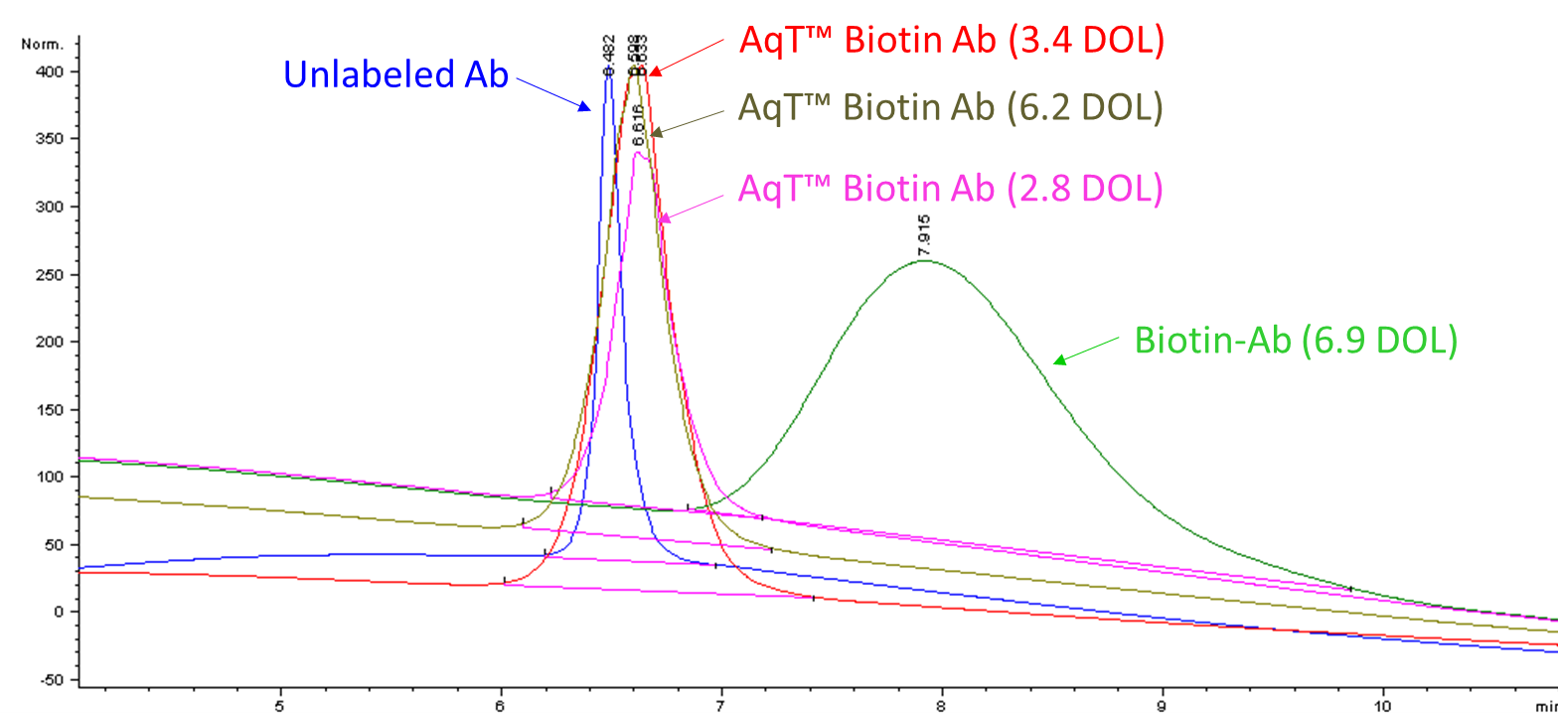
Figure 5. Hydrophobic interaction chromatographic (HIC) analysis of antibody, biotin-antibody (DOL: 6.9), and AqT™ biotinylated antibodies with various loading. AqT™ Biotin: Retain antibody properties
Biotin: Change antibody properties
|
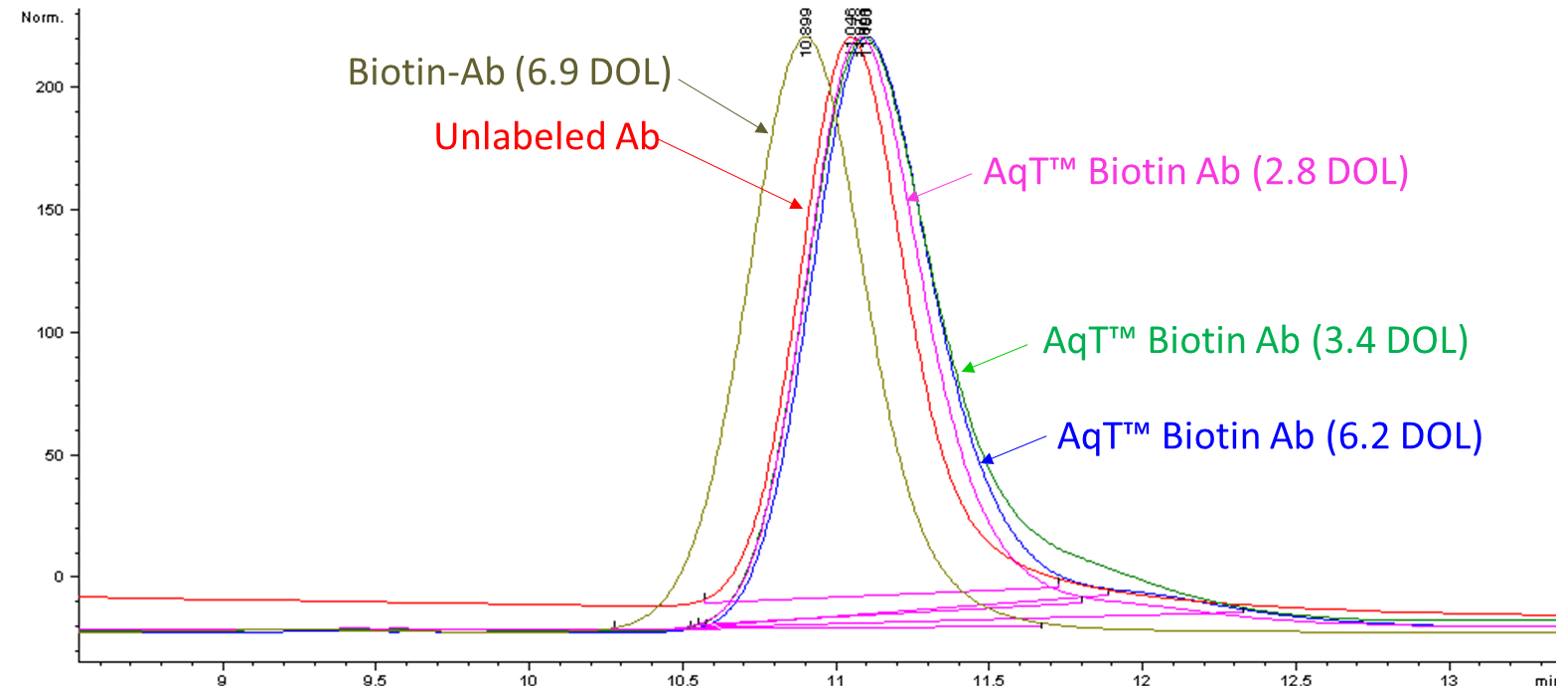 |
Figure 6. Size exclusion chromatographic (SEC) analysis of antibody, biotin-antibody (DOL: 6.9), and AqT™ biotinylated antibodies with various loading. AqT™ Biotin: Retain antibody properties
Biotin: Change antibody properties
|
Patent and license restrictions
AqT™ molecules are covered under WO2013/012961A2, US patent number 8907079B2, 9511150B2, US15/281,023, Chinese 201280034231.3, Australian 2012284055, Japan 2014-521744, and equivalent patents and patent applications in other countries in the name of CellMosaic, Inc. We are currently marketing limited AqT™ products for internal research and development.
The purchase of AqT™ products includes a limited license to use the AqT™ products for internal research and development but not for any commercial purposes. Commercial use shall include:
1. Sale, lease, license or other transfer of the material or any material derived or produced from it.
2. Sale, lease, license or other grant of rights to use this material or any material derived or produced from it.
3. Use of this material to perform services for a fee for third parties, including contract research and drug screening.
If you are interested in using AqT™ for commericial usage, please contact us for more information. For partnering and collaboration in the development of AqT™ therapeutics, please check our website at www.aqttherapeutics.com for more information.